Reference Interval of Serum Gastrin 17 (G-17) for Healthy Population: A Non-Invasive Screening Biomarker for Gastric Disorders
By Tayyaba Rashid1, Muhammad Dilawar Khan1, Hijab Batool1, Masood Afzal1, Omar Rasheed Chughtai2, Akhtar Sohail Chughtai2Affiliations
doi: 10.29271/jcpsp.2024.03.262ABSTRACT
Objective: To analyse fasting serum concentrations of G-17 in healthy individuals to establish the reference intervals (RIs) in the Pakistani population.
Study Design: Cross-sectional, observational study.
Place and Duration of the Study: Department of Clinical Chemistry and Immunology, Chughtai Institute of Pathology, Lahore, Pakistan, from October to December 2022.
Methodology: Fasting serum samples from one hundred and twenty healthy individuals between the age of 18-65 years were collected according to the CLSI recommendations after taking written informed consent. Samples were analysed on the auto-analyser for the quantitative measurement of serum G-17 by sandwich chemiluminescence immunoassay. Kolmogorov-Smirnov test was applied to check normality. A p-value of <0.05 was considered significant; 2.5th and 97.5th percentiles were computed using the formula 0.025 (n+1) and 0.0975 (n+1), respectively.
Results: Of the 120 samples, 74 were obtained from male patients and 46 from females. The mean age was 30.2 ±10.36 years. The histogram revealed a non-parametric distribution of the data. The established reference intervals by the rank-based method were 2.31 pg/mL and 49.36 pg/mL which corresponds to 2.5th and 97.5th percentiles, respectively. These were markedly different from the Chinese reference ranges.
Conclusion: Ethnic and geographic variations affect the trends of RIs of Serum G-17. There is a need to establish its population-specific RIs for G-17, so it can be used as a non-invasive option in identifying patients requiring invasive endoscopic intervention.
Key Words: Gastrin, Atrophic Gastritis, Biomarker, Reference values.
INTRODUCTION
Gastrin is a peptide hormone secreted by the G cells of the gastric antrum. The gastrin gene present on the 17q21 chromosome encodes the 101-amino acid precursor polypeptide hormone (preprogastrin).1 Post-translational modifications yield different biologically active peptides. About 95% of bio-active gastrin is α-amidated gastrin, of which 80–90% is G-17 and the remaining 5–10% is G-34.2 G-17 is the most effective form of the gastrin hormone, produced entirely by the antral G-cells making it a specific biomarker for assessment of the gastric antrum.
G-17 is involved in the secretion of gastric acid, proliferation, and differentiation of epithelial cells of the normal gastric mucosa.3 It also plays a role in gastric mucosa's repair and inflammatory response.
The circulating concentration of G-17 mainly depends on gastric pH and the number of G cells in the gastric antrum, levels typically increase after food stimulation, so a fasting sample is preferred.2 Serum G-17 levels are also influenced by the site and type of gastric lesion and Helicobacter pylori infection.3 Timely identification of gastric pathologies, especially atrophic gastritis, which is the premalignant lesion for gastric carcinoma, remains a problem, and diagnosis is usually made when the disease stage has already progressed to an advanced stage, and complete recovery is not possible.4 The gold standard investigation for diagnosing different gastric lesions is the gastroscopic and histological examination of gastric biopsy. Endoscopic examination is an uncomfortable, invasive, and costly procedure that usually lacks patient compliance.5 There is a lack of non-invasive screening modalities to identify gastric lesions.6 Serological markers such as G-17 can be used as a non-invasive option for the initial identification of different gastric diseases.
Serum G-17 levels within the reference range are suggestive of the normal physiology of the antrum.7 Chronic atrophic gastritis is an independent risk factor for gastric carcinoma, and the risk increases proportionally with the increasing grade and severity of gastric atrophy.6 In advanced or severe atrophic gastritis of the antrum, the fasting level of G-17 is low, and no increase will occur following protein stimulation. Hence basal levels of Serum G-17 can serve as an attractive non-invasive option to assess gastric function and structural integrity, as well as to identify the patients who require endoscopic examination.5,8,9
The use of reference intervals (RIs) helps clinicians in the confident interpretation of laboratory results.10 RIs are essential in the accurate and timely diagnosis of different disease processes. Population-based RIs need to be established or verified by every country for each parameter for proper clinical evaluation.11
Currently, the RIs and cut-off points of fasting serum G-17 levels for different gastric diseases are not established in the Pakistani population, thus affecting the potential clinical utility of this marker to be used as a non-invasive option in the initial evaluation of the structural and functional status of the gastric antrum. The objective of this study was to analyse fasting serum concentrations of G-17 in healthy individuals to establish the RIs in the Pakistani population.
METHODOLOGY
A cross-sectional study was conducted at the Department of Clinical Chemistry and Immunology, Chughtai Institute of Pathology, Lahore, Pakistan, from October to December 2022, after getting approval from the Institutional Review Board. Fasting serum G-17 samples from 120 healthy subjects aged 18-65 years were collected to establish RIs according to the CLSI recommendations.12 Individuals with the symptoms of peptic ulcer, gastroesophageal reflux disease, or any other systemic or infectious disease were excluded from the study. Smokers and individuals with a history of drug intakes, such as proton pump inhibitors and NSAIDs in the previous two weeks, were also excluded. Pregnant and lactating females were also not included. A purposive, non-probability sampling technique was used. Informed consent was taken from the individuals who fulfilled the inclusion criteria.
The samples were analysed for quantitative measurement of serum G-17 by sandwich chemiluminescence immunoassay (CLIA) on the chemistry autoanalyzer (Snibe Maglumi 800).13 Hemolyzed, lipemic, and icteric samples were rejected. Two levels of quality control were run with each batch.
Data were analysed using SPSS version 27. The data were assessed for normality by applying (the Kolmogorov-Smirnov test). A CI of 90% was used to establish RIs using non-parametric statistical methodology for 120 samples according to CLSI guidelines. A p-value of <0.05 was considered significant. Age and G-17 levels were expressed as mean ± SD and gender was described as n (%). The lower reference limits were estimated as the 2.5th percentile, and the upper limits as the 97.5th percentile of the distribution of test results for the reference population.10,14 2.5th and 97.5th percentiles were computed using the formula 0.025 (n+1) and 0.0975 (n+1), respectively.
RESULTS
Of the 120 samples, 74 (61.7%) were males, and 46 (38.3%) were females. The mean age was 30.22 (SD±10.36) years, and the mean fasting Serum G-17 levels were 18.53 (SD±11.57) pg/mL (Table I). Non-parametric statistics were applied as the histogram revealed a non-Gaussian distribution (Figure 1). Values were arranged in ascending order, followed by frequency distribution and ranking of the data (Table II), and the RIs based on the 2.5th and 97.5th percentiles established were 2.31 pg/mL and 49.36 pg/mL, corresponding to rank number 3 and 118, respectively (Table III).
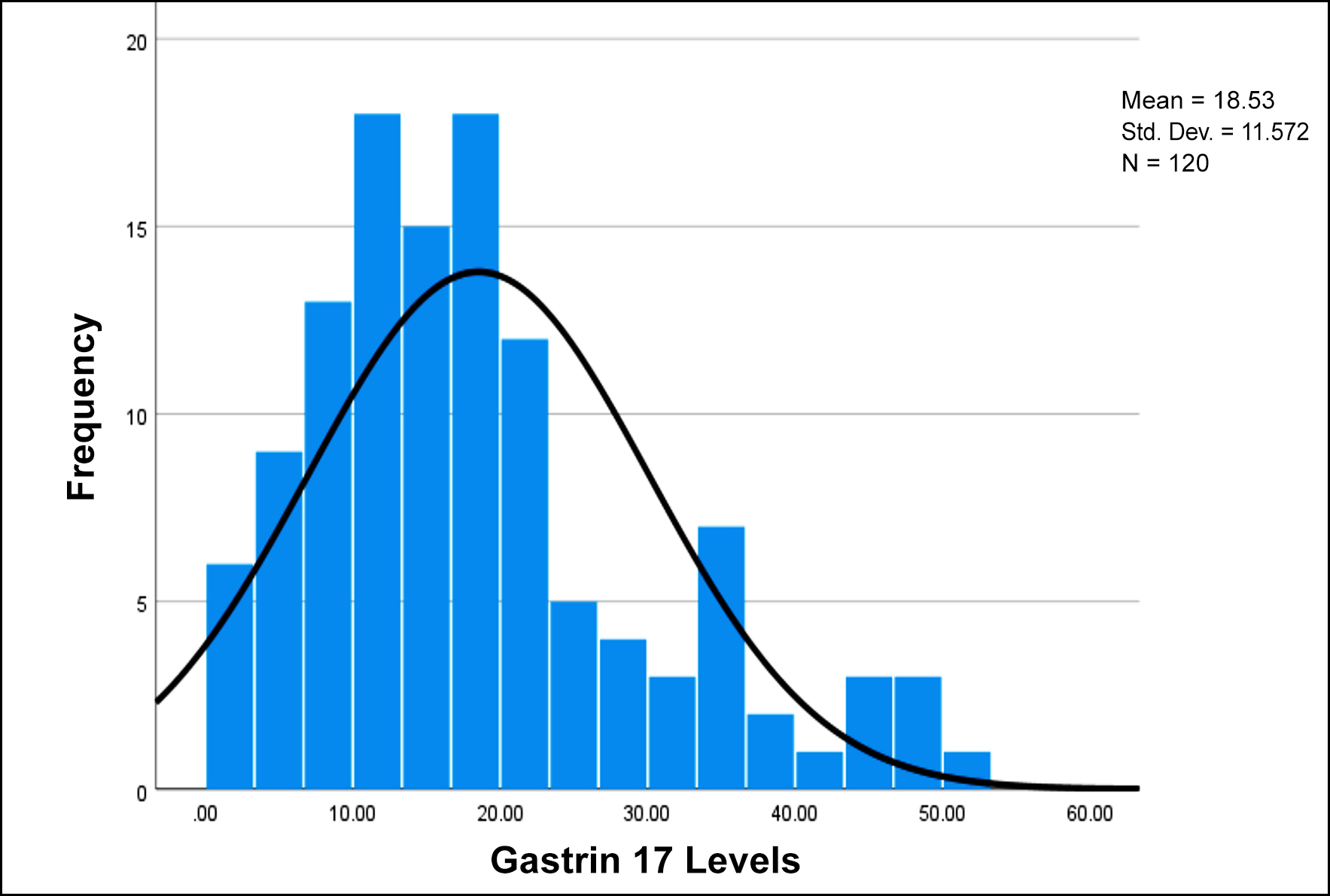 Figure 1: Histogram showing Gastrin-17 levels.
Figure 1: Histogram showing Gastrin-17 levels.
DISCUSSION
The diagnostic utility of the laboratory results depends on their interpretation which helps the clinicians to differentiate between health and disease states.15 Each laboratory should establish its RIs for each parameter according to the standard recommendations specific to the testing methodology used and the population covered by that particular laboratory.16 Establishing the RIs is expensive, complex, and time-consuming, and many laboratories cannot determine their RIs due to these constraints.15,16
To the authors’ knowledge, the RIs for fasting Serum G-17 have not been established for the Pakistani population until now. We aim to develop the RIs for this serum biomarker to be used as a screening and non-invasive element in identifying the patients requiring invasive investigations such as endoscopy to diagnose different gastric pathologies. Gastrointestinal diseases account for considerable healthcare use and expenditures.17 Gastric carcinogenesis is a cascade of events from various stages of atrophy to metaplastic and dysplastic changes eventually leading to gastric adenocarcinoma.6 Serum biomarkers seem to be an appealing option in diagnosing these premalignant lesions as an alternative to upper GI endoscopy with biopsy which is the gold standard but an invasive procedure.18
Table I: Descriptive statistics of fasting serum G-17 in healthy Pakistani population (n=120).
|
Variable |
Minimum
|
Maximum
|
Range |
Mean |
Median |
Standard Deviation |
|
G-17 (pg/mL) |
2.30 |
50.11 |
47.81 |
18.53 |
16.54 |
11.57 |
Table II: Ranking according to the levels of fasting serum G-17 (pg/mL).
|
Serum G-17 Levels |
Frequency |
Rank Number |
|
2.30 |
1 |
1 |
|
2.31 |
4 |
2-5 |
|
2.89 |
1 |
6 |
|
3.48 |
1 |
7 |
|
4.10 |
1 |
8 |
|
4.20 |
1 |
9 |
|
4.82 |
1 |
10 |
|
5.41 |
1 |
11 |
|
5.52 |
1 |
12 |
|
5.83 |
1 |
13 |
|
5.93 |
1 |
14 |
|
6.02 |
1 |
15 |
|
6.82 |
1 |
16 |
|
6.84 |
1 |
17 |
|
7.16 |
1 |
18 |
|
7.80 |
1 |
19 |
|
7.89 |
1 |
20 |
|
8.03 |
1 |
21 |
|
8.43 |
1 |
22 |
|
8.80 |
1 |
23 |
|
9.01 |
2 |
24-25 |
|
9.02 |
1 |
26 |
|
9.61 |
1 |
27 |
|
9.77 |
1 |
28 |
|
10.00 |
1 |
29 |
|
10.38 |
1 |
30 |
|
10.45 |
1 |
31 |
|
10.46 |
1 |
32 |
|
10.60 |
1 |
33 |
|
10.68 |
1 |
34 |
|
10.76 |
2 |
35-36 |
|
10.86 |
1 |
37 |
|
10.97 |
1 |
38 |
|
11.46 |
1 |
39 |
|
11.72 |
1 |
40 |
|
11.95 |
1 |
41 |
|
12.22 |
1 |
42 |
|
12.30 |
1 |
43 |
|
12.42 |
1 |
44 |
|
12.91 |
1 |
45 |
|
13.10 |
1 |
46 |
|
13.57 |
1 |
47 |
|
14.62 |
1 |
48 |
|
15.01 |
2 |
49-50 |
|
15.51 |
1 |
51 |
|
15.82 |
1 |
52 |
|
16.15 |
1 |
53 |
|
16.21 |
1 |
54 |
|
16.23 |
1 |
55 |
|
16.26 |
1 |
56 |
|
16.49 |
1 |
57 |
|
16.50 |
1 |
58 |
|
16.52 |
2 |
59-60 |
|
16.56 |
1 |
61 |
|
16.71 |
1 |
62 |
|
16.94 |
1 |
63 |
|
17.08 |
1 |
64 |
|
17.35 |
1 |
65 |
|
17.38 |
1 |
66 |
|
17.55 |
1 |
67 |
|
17.62 |
1 |
68 |
|
17.73 |
1 |
69 |
|
18.22 |
1 |
70 |
|
18.29 |
1 |
71 |
|
18.47 |
1 |
72 |
|
18.79 |
1 |
73 |
|
Continue… |
||
|
Serum G-17 Levels |
Frequency |
Rank Number |
|
18.90 |
1 |
74 |
|
19.03 |
1 |
75 |
|
19.22 |
1 |
76 |
|
19.25 |
1 |
77 |
|
19.42 |
1 |
78 |
|
19.89 |
1 |
79 |
|
20.01 |
1 |
80 |
|
20.40 |
1 |
81 |
|
20.47 |
1 |
82 |
|
20.49 |
1 |
83 |
|
20.58 |
1 |
84 |
|
20.86 |
1 |
85 |
|
22.47 |
1 |
86 |
|
22.57 |
1 |
87 |
|
22.69 |
1 |
88 |
|
22.91 |
1 |
89 |
|
22.98 |
2 |
90-91 |
|
23.58 |
1 |
92 |
|
24.53 |
1 |
93 |
|
25.46 |
1 |
94 |
|
25.51 |
1 |
95 |
|
25.63 |
1 |
96 |
|
27.41 |
1 |
97 |
|
28.31 |
1 |
98 |
|
29.21 |
1 |
99 |
|
29.34 |
1 |
100 |
|
32.16 |
1 |
101 |
|
32.22 |
1 |
102 |
|
32.35 |
1 |
103 |
|
33.67 |
1 |
104 |
|
33.70 |
1 |
105 |
|
34.51 |
2 |
106-107 |
|
35.56 |
1 |
108 |
|
35.98 |
1 |
109 |
|
36.35 |
1 |
110 |
|
37.62 |
1 |
111 |
|
37.65 |
1 |
112 |
|
41.71 |
1 |
113 |
|
44.46 |
1 |
114 |
|
44.84 |
1 |
115 |
|
45.73 |
1 |
116 |
|
47.05 |
1 |
117 |
|
49.36 |
1 |
118 |
|
49.46 |
1 |
119 |
|
50.11 |
1 |
120 |
Table III: Calculation of Rank number for the establishment of fasting Serum G-17 RIs.
|
|
Calculation of Rank |
Rank Number |
Levels of G-17 corresponding to Rank Number (pg/mL) |
|
Lower |
0.025 (120+1) = 3.01 |
3 |
2.31 |
|
Upper |
0.975 (120+1) = 117.97 |
118 |
49.36 |
Only a few studies have been done to establish the RIs for serum G-17. One such study conducted in China revealed that the levels of fasting serum G-17 were 0.8 - 3.9 pmol/L (1.68 - 8.18 pg/mL) in individuals with healthy stomachs.2 These results vary widely from the results of this study, i.e.,1.10 - 23.53 pmol/L (2.31- 49.36 pg/mL). The laboratory methodology used for the analysis of serum G-17 was ELISA. CLIA was used for the analysis of serum samples. The comparison with this study shows that RIs are affected by factors such as analytical assays, ethnic origins, living styles, population, and geographic differences.19 It is the need of the hour each laboratory should establish its RIs for the population being covered based on the specific testing method used at that particular laboratory.
Serum levels of G-17 can help detect pathological alteration in gastric antral mucosa and gastric atrophy at an early stage.20 The gastric antrum is the commonest site of gastric carcinoma, when there is mucosal atrophy, G cells disappear, leading to diminished secretion of G-17 even after the stimulatory factor such as protein load.18 The literature review reveals that G-17 and other gastric markers can be used as an initial screening tool in high-risk populations to identify the patients requiring endoscopic examination.21 This study is an initial step to establish RIs for Pakistani population to utilise this biomarker as a non-invasive, easy filter for the detection of premalignant pathologies in the stomach before proceeding to the invasive endoscopic examination.6
The present study covered a small population; there is a dire need for more extensive studies to establish RIs to be effectively used in the appropriate diagnosis and timely management of patients.
CONCLUSION
The RIs for fasting serum G-17 were established for a section healthy Pakistani population. The established RIs range from 2.31 to 49.36 pg/mL (1.10 - 23.53 pmol/L), which differ significantly from the RIs of the Chinese population references being used in Pakistani population. Ethnic and geographic variations affect the trends of RIs. Every laboratory should establish its reference intervals.
ETHICAL APPROVAL:
This study was approved by the Institutional Review Board of Chughtai Lab, Lahore.
PATIENTS’ CONSENT:
Written informed consents were taken from all the participants.
COMPETING INTEREST:
There is no conflict of interest to be declared.
AUTHORS’ CONTRIBUTION:
TR: Manuscript drafting, literature search, data collection, and analysis.
MDK: Study design, proofreading, and discussion.
HB: Statistical analysis, paper write-up, and proofreading.
MF: Sample collection and analysis, literature search.
ORC, ASC: Proofreading and finalisation of the study.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Schubert ML, Rehfeld JF. Gastric peptides-gastrin and somatostatin. Compr Physiol 2019; 10(1):197-228. doi: 10.1002/ cphy.c180035.
- Sun L, Tu H, Liu J, Gong Y, Xu Q, Jing J, et al. A comprehensive evaluation of fasting serum gastrin-17 as a predictor of diseased stomach in Chinese population. Scand J Gastroenterol 2014; 49(10):1164-72. doi: 10.3109/00365521. 2014.950693.
- Zhang Z, Sun L Ping, Gong Yh, Wang Xg, Zhang M, Yuan Y. Factors affecting the serum gastrin 17 level: An evidence-based analysis of 3906 serum samples among Chinese. J Dig Dis 2007; 8(2):72-6. doi: 10.1111/j.1443-9573.2007. 00288.x.
- Robles C, Rudzite D, Polaka I, Sjomina O, Tzivian L, Kikuste I, et al. Assessment of serum pepsinogens with and without co-testing with gastrin-17 in gastric cancer risk assessment-results from the GISTAR pilot study. Diagnostics (Basel) 2022; 12(7):1746. doi: 10.3390/diagnostics12071746.
- Zagari RM, Rabitti S, Greenwood DC, Eusebi LH, Vestito A, Bazzoli F. Systematic review with meta-analysis: Diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther 2017 Oct; 46(7):657-67. doi: 10.1111/apt.14248.
- Loong TH, Soon NC, Nik Mahmud NR, Naidu J, Rani RA, Abdul Hamid N, et al. Serum pepsinogen and gastrin-17 as potential biomarkers for pre-malignant lesions in the gastric corpus. Biomed Rep 2017; 7(5):460-8. doi: 10.3892/br. 2017.985.
- Syrjanen K, Eskelinen M, Peetsalu A, Sillakivi T, Sipponen P, Harkonen M, et al. GastroPanel® biomarker assay: The most comprehensive test for helicobacter pylori infection and its clinical sequelae. A critical review. Anticancer Res 2019; 39(3):1091–104. doi:10.21873/anticanres.13218.
- Chapelle N, Petryszyn P, Blin J, Leroy M, Le Berre‐Scoul C, Jirka I, et al. A panel of stomach‐specific biomarkers (GastroPanel®) for the diagnosis of atrophic gastritis: A prospective, multicenter study in a low gastric cancer incidence area. Helicobacter 2020; 25(5):e12727. doi: 10.1111/hel.12727.
- Nagasaki N, Takigawa H, Ito M, Boda T, Kotachi T, Hayashi R, et al. Diagnostic performance of the normal range of gastrin calculated using strict criteria based on a combination of serum markers and pathological evaluation for detecting gastritis: A retrospective study. BMC Gastroenterol 2023; 23(1):167. doi: 10.1186/s12876-023-02816-1.
- Ozarda Y. Reference intervals: Current status, recent developments and future considerations. Biochem Med (Zagreb) 2016; 26(1):5-16. doi: 10.11613/BM.2016.001.
- Bibi A, Haroon ZH, Shujaat A, Aamir M, Irum S, Jaffar SR. Establishing biotinidase reference interval: A foundation stone for newborn screening of biotinidase deficiency in Pakistan. J Pak Med Assoc 2022; 72(1):97-100. doi: 10. 47391/JPMA.2167.
- Henny J. The IFCC recommendations for determining reference intervals: Strengths and limitations / die ifcc-empfehlungen fur die Bestimmung von Referenzbereichen: Starken und Schwachen. J Lab Med 2009; 33(2):45–51. doi:10.1515/ jlm.2009.016.
- Orcun A, Yildiz Z, Koroglu Dagdelen L. Pediatric reference intervals for free testosterone, 17-oh progesterone, androstenedione, and IGF-1 with chemiluminescence immunoassay. Steroids 2022; 186:109078. doi:10.1016/j.steroids.2022.109078.
- Abebe M, Melku M, Enawgaw B, Birhan W, Deressa T, Terefe B, et al. Reference intervals of routine clinical chemistry parameters among apparently healthy young adults in Amhara National Regional State, Ethiopia. PLoS One 2018; 13(8):e0201782. doi: 10.1371/journal.pone.0201782.
- Placzkowska S, Terpinska M, Piwowar A. The Importance of Establishing Reference Intervals - is it still a Current Problem for Laboratory and Doctors? Clin Lab 2020; 66(8). doi: 10.7754/Clin.Lab.2020.191120.
- Ozarda Y, Higgins V, Adeli K. Verification of reference intervals in routine clinical laboratories: Practical challenges and recommendations. Clin Chem Lab Med 2018; 57(1):30-7. doi: 10.1515/cclm-2018-0059.
- Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology 2022; 162(2):621-44. doi: 10.1053/j.gastro.2021.10.017.
- Botezatu A, Bodrug N. Chronic atrophic gastritis: An update on diagnosis. Med Pharm Rep 2021; 94(1):7-14. doi: 10.15386/mpr-1887.
- Muneer S, Siddiqui I, Majid H, Jafri L, Humayun KN, Ahmed S, et al. Establishing reference interval for thyroid-stimulating hormone in children below two-year ages in Pakistani population. Ann Med Surg (Lond) 2021; 68:102601. doi: 10.1016/j.amsu.2021.102601.
- Shen H, Xiong K, Wu X, Cheng S, Lou Q, Jin H, et al. The diagnostic value of serum gastrin-17 and Pepsinogen for gastric cancer screening in eastern China. Gastroenterol Res Pract 2021; 2021:6894248. doi: 10.1155/2021/6894248.
- Xu Y, Miremadi A, Link A, Malfertheiner P, Fitzgerald RC, Bornschein J. Feasibility of combined screening for upper gastrointestinal adenocarcinoma risk by serology and Cytosponge testing: The sugar study. J Clin Pathol 2019; 72(12):825-9. doi:10.1136/jclinpath-2019-205700.